Evaluate cell activity levels (Brettanomyces,...) in addition to detection and quantification
Guarantee the quality and regularity of your great wines
EASY and SIMPLE: for use in the Winery
RELIABLE AND ACCURATE: all present populations and their physiological states
COMPLETE: data, and graphics
IMMEDIATE: compared to other analysis techniques

Wine-making monitoring by immuno-cytometry,
an available solution for everyone
In partnership with

Unique
on the market
Who is our monitoring solution designed for?
Owners
Make your production more reliable. Guarantee brand notoriety. Secure the value of your image and investments.
Technical direction
Control the quality of your production. Control the fermentations, aging period and barrels disinfection processes, as well as your blends. Secure your bottled products.
Oenological Laboratories
Offer a simple, reliable and fast solution to evaluate the risks of accidents or wine contamination. Reassure the wineries you advise.
Immuno-cytometry:
the solution for microbiological monitoring in real time

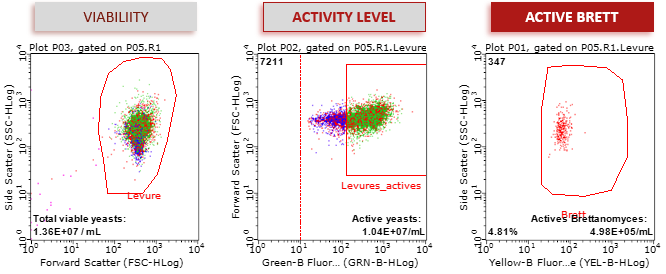
The immunological technique based on antibodies, asociated with flow cytometry, is the only method that permits to study the intact cell. Therefore, besides the populations specific detection (Brettanomyces, ...) and their absolute quantification, are available their health, their metabolic activity and their energy; their level of activity.
From the validation of the inoculum to the control of bottled wines, the wine-making monitoring by immuno-cytometry is the only solution that evaluates the real risks of fermentation accidents, wine alterations or barrels contamination. It permits avoiding prejudices to production quality, brand notoriety and the Winery's operating account.

1. Improve the wine quality
- Regain control of the quality and value of your wine
- Anticipated control of phenols appearance
- Optimise the use of sulphites (SO 2 )
2. Reduce risks
- Early evaluation of the activity of living yeast populations, particularly Brettanomyces
- Reduce the risk of fermentation accidents
- Reduce the risk of alterations during the ageing period and cross-contamination of blends
- Microbiological control of the pre-bottling and bottling processes


3. Improve the health and durability of your barrels stock
- Permits to preserve the quality and health of new barrels
- Early detection of several wines barrels contaminated by Brettanomyces
- Improve the barrels disinfection process
4. Decisions in less than 1 hour
- Get accurate and complete analysis results in less than 1 hour
- Don’t make blind decisions!
- Anticipate! Reduce systematic preventive and curative treatments.
- Improve significantly operating profitability

To implement internally or to outsource your analyses?
The choice between implanting in the Winery or outsourcing the analyses is based on an early, complete and rapid evaluation of the risks of accidents, alterations and contaminations of the wines, so that preventive or corrective measures can be taken immediately during all the winemaking process.
The solution offered by Q-Wine&Amarok Biotech provides, in less than 1 hour, information on the populations of yeasts, Brettanomyces or non-Brettanomyces, and bacterias present in the wines, as well as their levels of activity. It provides accurate data and complete graphs that are easy to use in the Winery.
By equipping their winery with the Q-Wine&Amarok Biotech solution, General and Technical managers will guarantee in total confidentiality the quality and regularity of the production, the health of the barrels park, and reduce significantly the operating costs.

Make an appointment with one of our experts
An expert will call you back within 48 hours to understand your needs and answer any questions you may have.
The main advantages of our solution
Security
Profitability
Quality
Rapidity
Confidentiality
Who are we ?

A heritage of trust and expertise for quality wines, thanks to more than 30 years' experience in the wine sector by our CEO and founder Patrick Paulian .

+

The partnership between Q-Wine Bureau and Amarok Biotechnologies is the alliance of Patrick Paulian and Vincent Genty’s expertise and experience, their knowledge of wine-making processes in the “field”, and their knowledge of microbiology and performance and risk assessment.
It is also their desire to provide the wine world with simple, and easy-to-implement control solutions to accompany the wineries and their managers on the path to excellence.

Patrick Paulian
Q-Wine Bureau
Patrick Paulian is a pioneer in the sale of oenotechnical solutions in Spain. It offers services or equipment; qualitative and profitable solutions that enable the Wineries to facilitate, guarantee and secure the production of their wines.

Vincent Genty
Amarok Biotechnologies
Vincent Genty, CEO and founder of Amarok Biotechnologies, is Doctor in Cellular and Molecular biology from more than 25 years.
In 2015, he developed the first anti-brettanomyces antibody for monitoring wine-making process by immuno-cytometry. His laboratory is ISO17025 accredited for evaluation of analytical performances of in-vitro diagnostic devices.
Frequently asked Questions
The immunological technique – the use of antibodies to specifically label a microorganism ( Brettanomyces in particular in wine), coupled with cytometry, is the only technique that keeps the cell membrane intact , it does not kill the cell, and permits therefore to have all its information in real conditions : physiological state, metabolic activity, energy, etc… ; its level of activity .
The presence of 10,000 dying yeasts/mL does not mean the same as that of 10,000 yeasts/mL in great shape! It is the level of activity of these yeasts which permits to guarantee the quality of an inoculum, the correct evolution of an alcoholic fermentation, or the evaluation of the real risk of the wines alteration and barrels contamination by Brettanomyces.
The volume needed to perform an immuno-cytometry analysis is 1 mL; however for the correct sampling in the cellar, it is preferable to work with 15 mL tubes. The very exhaustive monitoring of a batch from the start of vinification until the end of 12 months of aging does not represent more than 0.60L of wine.
In 25 minutes for a AMAROK BIOTECH LeviaTest, 50 minutes for a AMAROK BIOTECH BrettaTest and 35 minutes for a AMAROK BIOTECH TVO. Approximately 5 to 10 minutes of sample preparation, the rest being minor centrifugations and incubation times.
No. It only requires knowing how to handle a micropipette correctly; no special skills required.
Where you have 1.50 m of table. No need to work in a sterile environment.
It is profitable without any doubt!
The validation of the efficacy of the usual practices and the knowledge of the risks of alterations and contamination in less than 1 hour guarantee the quality of the production; it allows in the very short term a substantial reduction in the costs of systematic preventive and curative processes and treatments.
Neither the antibodies nor the reagents used are dangerous for human health; no need for lab gloves either.
Of course; it is even one of the fundamental objectives of the Monitoring Plan: to validate that the usual practices that you follow in the cellar are valid and effective.
Of course; with the AMAROK BIOTECH Leviatest , you have the 2 fundamental parameters to control the quality of the production of a fermenter: the level of activity of the viable yeasts and their vitality – ATP or Energy.
Within 24 hours, the AMAROK BIOTECH TVO_emb – total flora – confirms that the bottled wines following a sterile filtration process are compliant for sale.
Ask the opinion of our experts!
We will call you back within 48 hours to understand your needs and answer any questions.

- Our mission :
- To provide the wine world with high-quality, innovative and cost-effective control and process solutions to help to guarantee the excellence of great wines wine-making.